Why Do Lithium Batteries Get Worse Over Time?
Table of Contents
Lithium ion batteries get worse over time due to several factors that restrict ion mobility between the battery's electrodes. Normal charging and discharging cycles cause small amounts of damage to the battery's internal materials that restrict ion mobility over long periods of time. When a battery is overcharged, is run at too much current, is over discharged, or improperly stored this ion mobility issue will be more pronounced and a significant loss of capacity will be noticed.
Why Do Lithium Batteries Get Worse Over Time?
Lithium-ion batteries worsen over time primarily due to an SEI layer that forms after repeated charge and discharge cycles. When a lithium-ion battery is repeatedly charged and discharged, lithium ions get trapped in places they aren’t supposed to be, which alters the battery's internal structure. This SEI (solid electrolyte interface) layer forms and thickens, impeding ion movement. Also, external factors like operating or storing lithium batteries in high temperatures will accelerate these processes, which are all irreversible.
How Do Lithium Batteries Work?
To understand why lithium-ion batteries degrade over time, you have to first understand basic lithium-ion chemistry. A lithium-ion battery is made of two electrodes, which are called the anode and cathode. These electrodes are separated by a chemical electrolyte, which is non-conductive.
The battery generates power when lithium ions move from the anode to the cathode, which creates a flow of electric current. When the battery is recharged, the process happens in reverse, with lithium ions moving from the cathode back to the anode. This process is destructive.
So, Why Do Lithium-Ion Batteries Not Last Forever?
The largest contributing reason why lithium batteries worsen over time is due to their charging and discharging cycles. This is because every time a battery goes through a charge cycle (discharging and then recharging), small changes occur in the battery's structure. From one cycle to another, the amount of accrued damage is extremely small, but it adds up.
The lithium ions end up getting trapped within the microscopic structure of the electrodes, and that makes it so fewer ions can participate in the next charge cycle. Over a long period of time, a significant amount of ions become permanently trapped, which reduces the battery's overall capacity and increases its resistance. All of that being said there are things you can do to increase the cycle life of lithium cells, however, damage on every cycle is unavoidable.
If you are looking to minimize battery degradation check out our article on how to prolong the life of a lithium battery.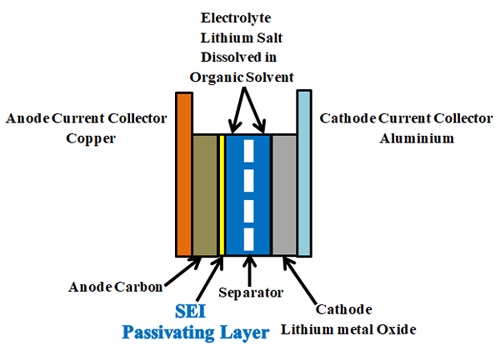
What Is the SEI Layer?
The SEI, also known as the Solid Electrolyte Interface, plays an important role inside the battery. This layer forms as the battery charges and discharges over time, with lithium ions interacting with the electrolyte to produce a thin, protective layer on the surface of the anode. It plays a vital role in the battery's overall stability and performance, especially in the initial stages of its life. A well-formed SEI layer prevents the electrolyte from further decomposing, so it actually helps the battery have longer longevity and higher safety.
These benefits, however, come with a caveat. As the battery goes through continuous charging and discharging cycles, the SEI layer tends to thicken. The continued formation and growth of the SEI layer lead to excessive consumption of lithium ions, which are crucial for the battery's performance. A thicker SEI layer essentially acts as a barrier, hindering the smooth transportation of these lithium ions between the anode and cathode. This manifests itself as a higher internal resistance and a lower capacity.
Impacts of High Temperatures and Overcharging
The naturally occurring SEI layer is not the only factor, however. There are external conditions like high temperatures that will accelerate lithium-ion battery degradation. This is because high temperatures work as a catalyst to increased ion activity, which inevitably leads to a thicker SEI layer. Overcharging and over-discharging can also lead to excessive lithium plating on the anode, which is another form of irreversible capacity loss.
What Other Factors Cause Lithium Batteries To Get Worse Over Time?
There are several other causes for the degradation of lithium-ion batteries that make less of an impact or are much less common but are still worth mentioning.
Mechanical Stress: Mechanical stress, such as vibration or physical impact, can damage the internal components of the battery. This can lead to the formation of cracks or disconnections within the electrodes or electrolyte, reducing the battery's overall capacity and efficiency.
Calendar Aging: Even when not in use, lithium-ion batteries degrade through a process called calendar aging. The passage of time, along with temperature and storage conditions, can cause chemical reactions within the battery that degrade its performance. Calendar aging can occur even if the battery is not being actively used but this happens very, very slowly.
Manufacturing Variations: Sometimes you just end up with a bad batch. Variations in the manufacturing process can affect the quality and longevity of lithium-ion batteries. Inconsistent electrode materials, impurities in the electrolyte, or inadequate cell assembly can all contribute to accelerated degradation.
Electrolyte Decomposition: During battery operation, the electrolyte, which is crucial for ion transport between the anode and cathode, can decompose over time. This decomposition results in the generation of gasses and the formation of unwanted compounds within the battery. These byproducts can further impair ion mobility and increase internal resistance, accelerating capacity loss.
Cathode Degradation: While the SEI layer formation predominantly affects the anode, the cathode also undergoes degradation. Transition metals in the cathode material can dissolve and migrate to the anode, contaminating it and contributing to capacity fade. Additionally, structural changes in the cathode material, such as phase transitions and mechanical stress, can reduce its effectiveness in lithium-ion storage.
Formation of Lithium Plating: Lithium plating occurs when lithium deposits on the anode surface, rather than intercalating into the anode material. This phenomenon is more likely to happen during high-rate charging, low-temperature operations, or overcharging. Lithium plating not only reduces the amount of lithium available for cycling but also poses a safety risk, as it can lead to the formation of dendrites that may cause short circuits.
Particle Fracturing: Repeated cycling can cause the active material particles within the electrodes to fracture and disintegrate. This mechanical degradation reduces the effective surface area available for lithium-ion intercalation and deintercalation, thereby decreasing the battery’s capacity and increasing resistance.
The causes of worsening lithium batteries are due to several factors that restrict ion mobility and alter the internal structure of the battery. The primary reason is the formation and thickening of the SEI (Solid Electrolyte Interface) layer during repeated charge and discharge cycles, which traps lithium ions and increases internal resistance. Additional reasons why lithium batteries degrade include high temperatures, overcharging, over-discharging, and mechanical stress, which accelerate degradation by causing further structural damage or excessive lithium plating. Calendar aging, even when batteries are not in use, and manufacturing variations can also contribute to the reduced longevity and efficiency of lithium-ion batteries.


