Understanding Reverse Charging: How It Happens and Why It Destroys Batteries
Table of Contents
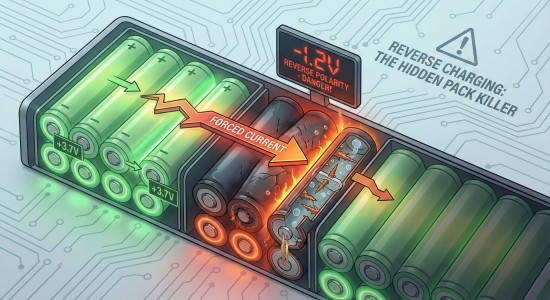
Reverse charging happens when a cell or cell group in a battery pack becomes deeply discharged while the others continue to drive current through that cell or cell group. When you build a battery pack, you connect the cell groups together to achieve a higher voltage.
If one of the cells falls to zero volts, the battery pack can still run. If the BMS is faulty and does not cut the battery pack off, or the BMS discharge has been bypassed, or the BMS has been foregone altogether, either way, if you continue to run current from that battery, the cell will go all the way down to zero and then it will continue going in that direction past zero into the negative, effectively reversing its polarity.
What Happens When You Reverse Charge a Cell
So what happens when you reverse charge a cell? What actually happens to the cell itself? Well, you get irreversible damage that happens from the cell. The chemistry that makes all this stuff work is not very stable. It only has a range of stability that exists under certain circumstances, and one of those factors in those circumstances is the running voltage of the cell.
At very low voltages of around 2.6 volts or less, the anode potential rises closer to or above zero volts. When that happens, the copper from the anode's current collector starts to dissolve into the electrolyte as copper ions. Then, when the cells recharge, those copper ions can replate irregularly onto the anode or cathode, forming metallic dendrites. The dendrites can then pierce the separator, causing an internal short circuit and potential thermal runaway.
In these circumstances, when you drive the chemistry to such a low voltage, gases are generated and swelling can happen. This is just about one of the only ways you're going to find 18650s and 21700s swollen like LiPo batteries and prismatics can be.
Accelerating Effects and Heat Generation
These effects accelerate more and more as the voltage falls. Under normal circumstances, when the cell is by itself, as the voltage falls, its ability to carry current also falls because, as we know, all of those things are related. But if the cell itself is not having to produce the power, and the power is coming from another source, and it simply has to go through the cell, then you end up in a really dangerous situation.
Now, instead of heat being produced in the cell as a function of its internal series resistance (which just causes a small voltage drop under load), the heat being produced inside the cell is a result of ohmic losses, just like any other resistor. That means the amount of heat is going to be proportional to the flowing current. Like a resistor, that cell is going to get extremely hot.
Anytime the cell goes below two volts for more than a few hours, you really don't even want to try to recover it. Honestly, at that point, it's not something that you're going to want to put in a battery pack. But once they go negative voltage, as in below zero, you absolutely, most certainly, do not want to use them, no matter how short of a time period they spent in those conditions.
Spotting the Problem Before It Happens
So how can you spot this problem before it happens? For this to happen, you have to have a cell group or cell groups that have not only a lower voltage than the other cell groups but a much, much lower voltage than the other cell groups. You also have to have a BMS that is either not present, malfunctioned, or bypassed for this to happen.
Even still, you can see this kind of thing potentially coming if you notice a cell group lagging far behind another cell group consistently. That means this kind of thing is either well on the way or it's about to be prevented from happening by your BMS.
Preventing Reverse Charging
What can you do to prevent this from happening? You can use a quality BMS that simply wouldn’t let this happen — that’s number one. But it's possible that the BMS is a quality BMS, and for some freak incident, it has failed. So, to prevent this from happening, you want to keep the battery as balanced as much as you possibly can. That’s about all you can do.
But the thing is, the BMS is responsible for that balancing. So the very device that would be required to fail for this to become an issue is the device that you're going to need to rely on to prevent this issue from happening in the first place.
Of course, whether the BMS fails to allow this to happen or not is separate from the fact that the cells have to fail in the first place. So the best way to avoid this is to build or buy a high-quality battery that uses high-quality cells that are in the right configuration, built by somebody who has a deep understanding of what's going on inside of a battery pack.
Conclusion
Reverse charging is literally the process of charging a battery backwards, sending negative in the positive and positive in the negative. There are two main paths for this two happen. One way is to hook a cell up backwards and charge it backwards, which almost no one is going to do. Another way reverse charging happens is when a cell group fails within a battery that either has a failed or bypassed or non-existent BMS.
We hope this article helps you learn everything you need to know about reverse charging. Thank you for reading.