What Are The Main Types Of Batteries Used Today?
Table of Contents
Battery Categories
You can put any given battery in one of two categories. It’s either a primary battery or a secondary battery. A primary battery is a disposable or non‑rechargeable battery, while a secondary battery is a reusable or rechargeable battery. At first glance, you might think a primary battery is a rechargeable battery, but that’s just because we are from this era where rechargeable batteries have finally become good enough to be the most common kind of battery for many applications, so it just seems like they should be the 'primary' ones.
Primary Batteries
When it comes to primary batteries, we still use alkaline, zinc carbon, and lithium non‑rechargeable batteries. Pretty much all the other chemistries have gone by the historical wayside. Alkaline is the most popular type of primary battery, while zinc batteries are still used in developing parts of the world due to their extremely low cost.
Alkaline
Alkaline is still widely used in household devices like remote controls and flashlights. It’s extremely low‑cost. It has a very long shelf life. They can last up to 10 years, and they have a relatively stable voltage under load. They’re obviously non‑rechargeable, so you pay for them once and use them once rather than paying for them once and using them over and over again.
Zinc Carbon
In some parts of the world and in some niche applications, zinc carbon batteries are still used. It’s an older technology, and it’s generally only found in low‑drain devices. It’s extremely cheap to manufacture, which is pretty much the only reason it’s still used, but they have a very low capacity and their voltage drops pretty tremendously under even moderate loads, and they have a shorter shelf life.
Lithium (Non‑Rechargeable)
Another type of primary battery that’s become increasingly popular lately are lithium batteries. Usually we associate lithium batteries as being rechargeable, but in this case they aren’t. They are used in high‑drain or extreme‑temperature applications like in cameras, sensors, etc.
They have extremely high energy density, a very long 10‑plus‑year shelf life, and a wide operating‑temperature range; but they are more expensive, and they have some safety considerations, as you’d expect with any other lithium‑ion battery technology, because they have such a low resistance that they can deliver a tremendous amount of current under short‑circuit situations.
Secondary Batteries
Now we’ve come to the secondary batteries, which are also known as rechargeable batteries.
Lead Acid
We still use lead-acid batteries in many applications. They’re still dominant in the automotive industry and they’re used as starter batteries. They’re still used as backup batteries in small-scale UPSs, which are uninterruptible power supplies, and some people still use them for off-grid solar, although you can make a pretty clear point that the total cost of ownership is actually higher with lead-acid batteries in that regard. Lead acid batteries have a very low cost per kilowatt-hour and they are extremely reliable. If you’re considering a retrofit, check out our guide on How To Replace Lead Acid/AGM With Lithium to see the potential benefits and pitfalls of moving to a lithium-based system.
Lead-acid batteries are extremely heavy, very low-energy-density, and contain large amounts of lead that have to be contended with one way or the other.
Nickel Cadmium
Nickel‑cadmium batteries are still in use in some very old devices. There’s plenty of drills and things out there that were designed to run on nickel‑cadmium batteries, and they’re still working. They can tolerate extreme temperatures, and they have very high discharge rates. A major con of NiCd is the memory effect, and cadmium is very toxic; they’ve largely been phased out by nickel‑metal‑hydride.
Nickel Metal Hydride
Nickel‑metal‑hydride technology is still used in rechargeable AAs and AAAs and in some hybrid vehicles like the older Toyota Prius. NiMh batteries have a higher capacity compared to NiCd and less of a memory effect, but they still have a memory effect.
They have a relatively high self‑discharge rate compared to other battery technologies and only a moderate energy density.
Lithium‑Ion Rechargeable Batteries
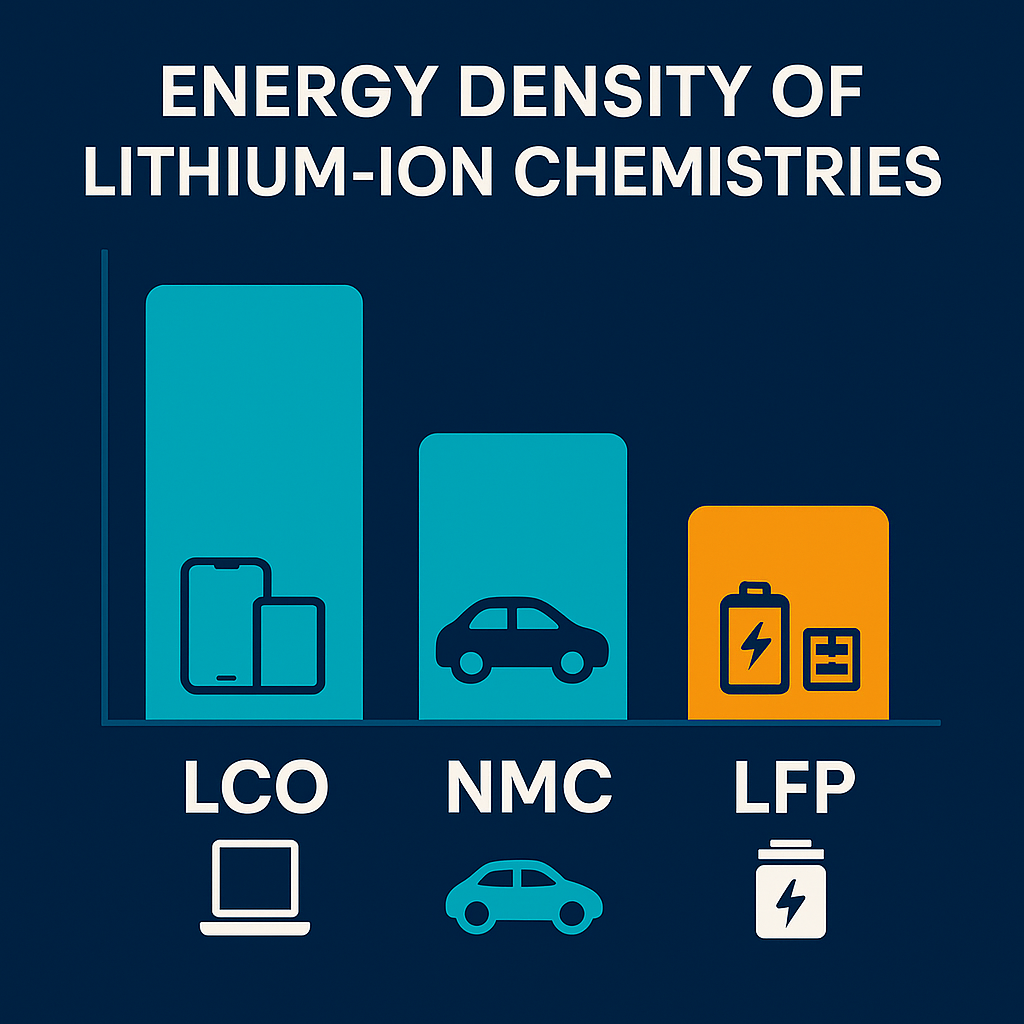
And now, of course, that brings us to the most popular type of battery of all time: the lithium-ion rechargeable battery. This thing is ubiquitous in consumer electronics such as smartphones, laptops, electric vehicles, and more. And you can even find it in industrial applications like grid-storage systems. There are many types of lithium-ion chemistries, but the three most popular are LCO, NMC, and LFP. If you’re looking to build your own pack from scratch, take a look at our step-by-step walkthrough in Building A Lithium Battery Pack From 18650 Cells.
There are many types of lithium‑ion chemistries, but the three most popular are:
- LCO (lithium cobalt oxide): Known for its extremely high energy density, and it’s used most of the time in phones and laptops.
- NMC (lithium nickel manganese cobalt oxide): A close relative of LCO, it offers the best balance of energy, power, and longevity, and it’s mainly used in electric vehicles, phones, drills, RC applications, and more.
- LFP (lithium iron phosphate): An up‑and‑coming chemistry with a much longer cycle life than the other two lithium‑ion chemistries; it’s much safer, and it finds the best use in stationary storage applications and some EVs that have the space and are efficient enough to not need a lot of capacity.
Why are batteries called AA, AAA, C, and D?
At first, the battery names only went up in size. With A being the smallest battery, B being a little larger, and C even larger than that, and so on. But as technology progressed and devices and the batteries powering them actually ended up getting smaller, the only option was to use more As to signify than the new types were smaller than A. You can see a similar situation of wire gauges, its why we have 00, 000, etc.
What Is The Most Common Type Of Battery Used Today?
Lithium‑ion batteries are the most common type of battery used on earth today. This is because lithium ion batteries have such a high energy density, a high single cell voltage compared to other battery types, a low self‑discharge rate and no memory effect. They also have a long cycle life compared to other rechargeable batteries. Lithium ion batteries can be charged quickly, they’re extremely scalable and have form‑factor flexibility in their construction, and everybody’s making them right now. So the costs are coming down through economies of scale, and the battery‑management systems used to govern them have become extremely sophisticated, reliable, and low‑cost over time.
Well, that pretty much covers it for what are the main types of batteries used today. We hope this article helped you learn more about the portable power landscape, thanks for reading!